Quality Assurance in the Medical Device Production Environment - Environmental Control and Identification of Microorganisms

by Arnaud Carlotti: President, Eurofins IDmyk
Guillaume Ledoux: Utilities Technical Expert, Eurofins
Vincent Rietsch: Head of Medical Devices, Eurofins
Bio-contamination of medical devices is a major public health problem, especially for Class IIa, IIb and III devices. The safety of the device for patients is the full responsibility of the manufacturer. Strict control of the production environment (air, surfaces), but also utilities used (water, gas, lubricants), are required to minimize the adventitious contamination of medical devices.
The implementation of an environmental control plan is done in addition to the qualification phase of premises and utilities. During the initial qualifications, the selection of the relevant control points (usually the worst cases) is carried out, as well as a quantitative and qualitative mapping of the bacterial and fungal species present.
This initial inventory will allow the detection and identification of subsequent microbiological changes in production conditions during subsequent routine checks to keep the manufacturing process under control.
In the case of compulsorily sterile medical devices (free of any viable microorganisms), the validation and control of sterilization processes involves a study of the initial bioburden and the use of bio-indicators (worst-case).
In the case of reusable and refurbished devices, the validation requirements vary according to the criticality of the device type with respect to the infectious risk.
Bioburden is the population of viable microorganisms present on or in a product and / or sterile barrier system. It comes from the environment, utilities, operators and raw materials. The quantitative and qualitative characterization of this bioburden is an integral part of the controls.
Quantitatively, the count of microorganisms present (expressed either in number of cells or in colony forming units per unit area or volume) is performed according to different methods to ensure compliance with specifications (ex. <50 CFU / g).
For example, for air bio-contamination, bio-impactors are used to collect a determined volume of air and to impact the cells suspended on agar culture medium. After incubation under the appropriate conditions (eg 30-35 ° C / 5 days on TSA medium for bacteria or 20-25 ° C / 5 days for fungi on Sabouraud medium), the colonies formed are counted and brought back to unit of air volume collected.
For surface biocontamination, microbiological samples are taken by swabbing under standardized conditions or by contact with an agar medium (eg contact agar).
Process waters are analyzed, for example, by filtration of a determined volume (eg 1 mL or 100 mL to cover different concentrations of contaminant, from the most concentrated to at least concentrated). The filter is then sterilely transferred to the surface of a solid culture medium (eg R2A medium well adapted to water bacteria). This is then incubated to allow the development of colonies of microorganisms to be counted.
These conventional techniques only detect viable and cultivable bacteria. The use of culture media for the growth of the widest variety of bacteria is therefore essential.
Specific research of bacterial or fungal species is sometimes possible using "chromogenic" solid culture media. In fact, they contain chromogenic substrates which, when specifically degraded by a growing bacterial species, cause colony coloration and thus their presumptive identification (eg chromed medium Burkholderia Sepacia group whose colonies appear pink).
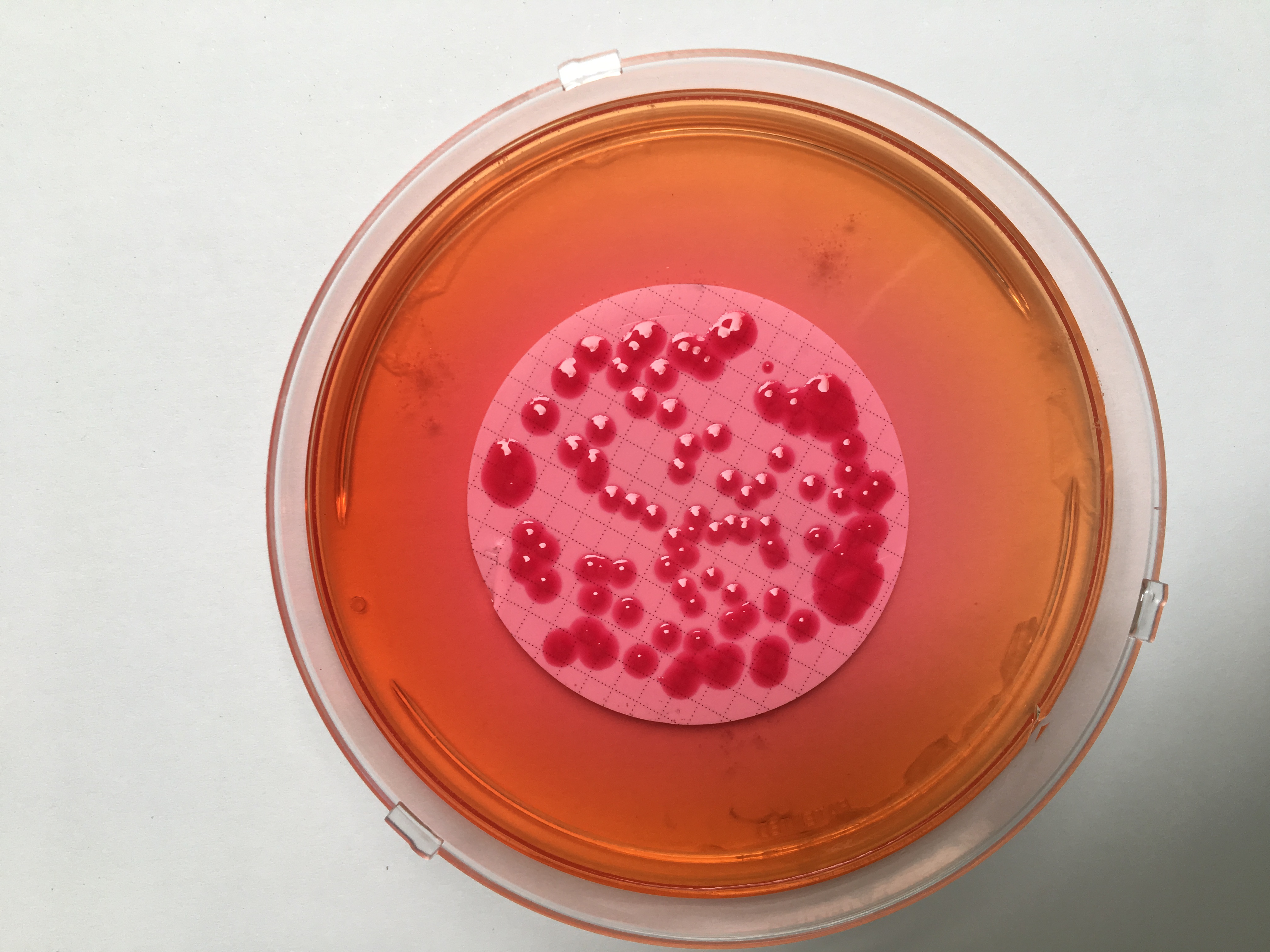
Figure 1: Characteristic appearance of B. cepacia colonies
on CIBC chromogenic media (bioMerieux)
Flow cytometry analysis methods (liquid or solid) are alternatives for counting both viable and non-viable microorganisms.
For gases, analyzes require special equipment and sampling conditions (bio-collectors, pressure-reducing systems) but the microbiological techniques are identical to those described above.
In terms of quality, species identification of micro-organisms in the environment and bioburden can be crucial.
For example, in the case of a water loop that meets quantitative specifications, the presence of the virulent pathogenic species Burkholderia Sepacia is unacceptable.
As part of the sterilization of medical devices, it is the meaning of ISO 11737-1 (2018) that states that quantitative analysis alone is not sufficient, but needs to be supplemented by qualitative analysis. , i.e. identification of present species. Different methods of identification are available for bacteria and fungi that provide a reliable and accurate result in 2 to 3 days (e.g. comparative sequencing, mass spectrometry).
In conclusion, quality control of the production environment requires quantitative but also qualitative microbiological controls through the identification of present species.
Click to view the full Device Med, October 2018 article.
















































